The Beam Team
Related Articles
Graphic: How Crystallography Maps the Structure of a Protein
Carla Reiter is a writer, editor and translator in Chicago.
Tell us what you think. E-mail comments or questions to the editors at letters@northwestern.edu.
Ever wonder about those strange designations we use throughout Northwestern to identify alumni of the various schools of the University? See the complete list.
Find Us on Social Media
At the Advanced Photon Source, Northwestern researchers use extremely bright X-rays to do atomic-level basic research that is critical to the creation of new drugs, innovative catalysts, better batteries and new sources of energy.
It could be a room in any midrange commercial hotel. There are blackout curtains, tea-scented soaps and body lotions, and a flat-screen TV — all standard issue.
But this is the Guest House at Argonne National Laboratory, 25 miles southwest of Chicago. And if you scroll through the TV offerings, you discover something a bit different: There are five channels dedicated to “the beam.”
The beam is an intense concentration of X-rays produced at Argonne’s Advanced Photon Source synchrotron and made available to scientists for research.
On a muggy evening last summer, the Beam Status Channel informed guests that the beam was “delivered” and that 59 beamlines were operating, meaning that 59 different research groups had people on the experimental floor.
On any given day or night, Northwestern scientists and graduate students make up a hefty percentage of those research groups. The beam is a vital tool for scientists seeking to make discoveries leading to new drugs, catalysts and electronic devices and to design clean new sources of energy.
“These are the brightest, most intense X-rays available in the Western Hemisphere,” says Michael Bedzyk, professor of materials science and engineering at the McCormick School of Engineering and Applied Science and co-director of the Northwestern Synchrotron Research Center. Bedzyk’s own group uses the X-rays to explore how atoms align at the interfaces that separate two different materials.
Bedzyk is one of more than 280 Northwestern faculty members who applied for beam time for himself and his students and postdoctoral fellows in 2013. In addition to Bedzyk, other frequent users of the beam include many of the University’s top scientists in biology, chemistry, engineering, materials science, medicine and physics: Wayne Anderson, Robert Lamb, Tobin Marks, Chad Mirkin, Alfonso Mondragón, Justin Notestein, Thomas O’Halloran, Aaron Packman, Amanda Petford-Long, Heather Pinkett, Sarah Rice, Amy Rosenzweig, Sam Stupp and Gayle Woloschak.

Members of the DuPont-Northwestern-Dow Collaborative Access Team and Life Sciences Collaborative Access Team, the two groups run by the Northwestern Synchrotron Research Center at the Advanced Photon Source. See the list of team members in the photograph. Photo by Jim Prisching.
The Advanced Photon Source, which operates 24/7, is shared by more than 5,000 users from industry, academia and government laboratories around the world. Since the facility came online in 1996, researchers have been using the X-rays for basic and applied research in numerous scientific disciplines.
“The Advanced Photon Source allows our faculty, graduate students and postdocs to have ready access to one of the best light sources on earth,” says Jay Walsh, Northwestern’s vice president for research.
“The fact that it’s only an hour away from Evanston is just phenomenal.”
Students usually commute by carpool.
“There’s a Google calendar, and the students manage the schedule,” adds Walsh, whose office provides cars for students. “Those cars are always full going back and forth between Argonne and campus.”
The grad students who aren’t in the driver’s seat often catch up on sleep on the trip because once they arrive at the APS, they face an intense workload.
“People take a 12-hour shift, and then the next two people take a 12-hour shift,” says Leah Shoer, a chemistry graduate student who worked as part of a four-person team at the APS.
“It’s kind of bad luck if you draw the night shift,” she says. But, she adds, being on the “Beam Team” has a special cachet. “It has a little bit of an air of mystery.”

The Advanced Photon Source dominates the 1,500-acre Argonne National Laboratory campus. Courtesy of Argonne National Laboratory.
The Synchrotron
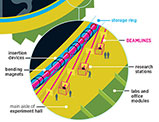
Seen from the air, the Advanced Photon Source dominates Argonne’s 1,500-acre woodland and wetland campus near Lemont, Ill., in southeastern DuPage County. The APS is one of the three largest synchrotron light sources in the world.
The ring is nearly three-quarters of a mile in circumference, or big enough to encircle a Major League Baseball stadium — which is why researchers often transport equipment around the ring using a fleet of colorful tricycles with wire baskets on the rear.
The X-rays are created in the APS synchrotron, an accelerator that speeds electrons around a ring. When they travel at close to the velocity of light, electromagnets shunt them into a gigantic storage ring. The electrons emit radiation in the form of X-rays as they curve around the ring. That X-ray beam is focused down to a small diameter and directed into the tangent beamlines that feed the experimental bays. The very short wavelengths of the X-rays are on more or less the same scale as the atoms found in the molecules of cells and many materials, or the distances between atoms in crystalline structures. This means that these hard X-rays — high-energy X-rays that can traverse relatively thick objects without being much absorbed or scattered — are uniquely well suited for use as a probe of these structures at a resolution down to the atomic level.
As colossal as it is, the APS is essentially a microscope — the very, very large being used to illuminate the very, very small in a way few other instruments can.
“If you’re doing any kind of science that requires you to understand the structure of materials and how those structures change with time, then the Advanced Photon Source really is a critical state-of-the-art instrument,” says Michael R. Wasielewski ’05 P, ’07 P, Clare Hamilton Hall Professor of Chemistry at the Weinberg College of Arts and Sciences and director of the Argonne-Northwestern Solar Energy Research Center.
The APS is used in three ways. “First,” explains Walsh, “it takes images. You can take pictures inside engines, for example, so you can watch combustion happen and develop better fuel injectors.
“Second, it can measure the patterns of waves bouncing off materials. If you’ve ever flown over Lake Michigan and looked at the waves off the intake cribs, we do the same thing with X-rays. You bounce waves off materials and then you can characterize the materials.
“Third, it’s used for spectroscopy. Like a black light at a disco, where the ultraviolet light makes everybody’s white shirt glow in the dark, you can shine the X-rays on things, and the light fluoresces at lower X-ray energies, which tell you what chemical elements, or atoms, are present in the material being X-rayed.”
Northwestern & the Advanced Photon Source
Northwestern has been involved with the Advanced Photon Source since the early 1980s, when the University helped write the original proposal to the U.S. Department of Energy to help Argonne get the facility built.
In concert with Dow Chemical Co. and DuPont, Northwestern, led by former McCormick dean Jerry Cohen, won a bid to build and equip one of the first four experimental sectors along the circumference of the APS ring. Today there are 34 such sectors. And the University’s Synchrotron Research Center operates two of them: The DuPont-Northwestern-Dow Collaborative Access Team (DND-CAT) focuses on materials science; and the Life Sciences Collaborative Access Team (LS-CAT) focuses on life sciences. Northwestern scientists, however, use the beam in other sectors as well.
Any scientist, whether a Nobel laureate or first-year physics student, can write a proposal to use the APS. If the proposal is accepted, use of the facility is free, as long as the results of the research are published openly in the scientific literature. Companies can and do use the APS for proprietary work, but they pay a fee.
Each beamline sector has its own unique instruments and Argonne personnel with the expertise to run them, gather data and collect information from that data.
There are 66 experimental bays with simultaneous access to the beam. And researchers can instrument and tune the beam in their bay to the characteristics best suited to their experiments.
Basic Research for Drug & Vaccine Discovery
At LS-CAT the focus is biological. “The beam allows people to look at the structure of molecules — proteins and nucleic acids — and see how they’re made, where the atoms are,” says Alfonso Mondragón, a professor of molecular biosciences in the Weinberg College and co-director of LS-CAT.
Mondragón’s group seeks to understand the structure of a special RNA molecule that has the ability to catalyze chemical reactions inside a cell. “Without the structure we don’t understand what’s going on,” he says. “The structure serves as a very powerful guide to other types of experiments. So it really is a way to understand at the atomic level how proteins and nucleic acids work.”
Those proteins include disease-causing pathogens such as anthrax, cholera and salmonella. Wayne Anderson, a professor of biochemistry and molecular genetics at the Feinberg School of Medicine, heads a group using the LS-CAT beam to create a kind of reference library of the 3-D structures of proteins of such pathogens.

Feinberg School of Medicine professor Wayne Anderson holds a protein crystal sample in the X-ray beam path of a detector. Courtesy of Argonne National Laboratory.
“Our work is a combination of basic biology questions and things that are the groundwork for translational research for drug and vaccine discovery,” he explains. The Center for Structural Genomics of Infectious Diseases, a collaboration between Northwestern and Argonne, makes the protein structures available to the scientific community at no cost. Some of the proteins may be good candidates for use in developing drugs. Others are potential vaccines. And still others provide insight into biological processes, such as how a pathogen interacts with the host organism and manipulates its biology.
3-D Atomic Maps of Proteins
Knowing a pathogen’s 3-D structure lets scientists infer what kinds of compounds might be able to fit into it and stop it from working: in other words, a drug.

Valerie Tokars maps protein structures in the development of compounds for possible pharmaceuticals.© Northwestern Memorial Hospital/Laura Brown.
Valerie Tokars, assistant professor of pharmacology at Feinberg, likens the picture the beam gives to a puzzle with some of its pieces missing. “You can see the space that’s available for pieces to fit,” she says. “Once you start to see what’s available, there are certain pieces you rule out because you know they’re not going to fit — they’re not even worth trying.”
Scientists make the map of a biological macro-molecule using a technique called X-ray crystallography. First, they must induce their proteins or nucleic acids to form a crystal — often not an easy task. “Crystallizing them is a bit of an art,” says Mondragón. “Some molecules are recalcitrant.” The crystal samples go into a liquid nitrogen vat in the LS-CAT experimental bay. It is crucial to keep them as cold as possible, “so they don’t cook in the beam,” explains Keith Brister, operations manager at LS-CAT. “And even then, they don’t last long.”
The samples are miniscule — on the order of 50 microns on each side, or about the diameter of a human hair. A robotic arm plucks each sample from the vat and positions it in the path of the beam.
When the X-ray beam hits the atoms that make up the crystal, it bounces off each of the electrons in each atom, sending out waves — like a wave hitting a pier in a lake. In a regular array of atoms like a crystal, the waves interact. Most cancel each other out, but others build on each other in ways scientists can calculate.
A detector registers the places where the waves add to each other as a pattern of dots: a diffraction pattern. “From that you can find out what the crystal looks like in atomic detail,” says Mondragón.
“When I started doing this 45 years ago, we used an X-ray generator in the laboratory, and it took years to determine one protein structure,” says Anderson. “Now we average two a week.”
The ability to get rapid feedback from one’s data is crucial to a Northwestern group using LS-CAT to develop a novel treatment for Alzheimer’s disease. The project is a collaboration that includes Tokars and Anderson, as well as D. Martin Watterson, the John G. Searle Professor of Molecular Biology and Biochemistry at Northwestern, and scientists at Columbia University in New York.
The group is targeting a type of enzyme called a protein kinase. They are focused on a particular kinase that normally helps regulate the transmission of signals between cells in the brain, but when it’s overactive, it disrupts that communication.
Tokars grows crystals of the kinase in her Chicago lab, incorporating molecules of the drug candidate into the crystal lattice. Then she takes the kinase-and-drug crystals to Argonne and tests them in the beam.
With the map produced by the diffraction data, Tokars and other scientists refine the candidate drug, designing the compound to fit into the spaces between atoms. Feedback from the beam greatly streamlines the refining process. “It really helps you to prioritize and minimize cost and time,” Tokars says.
The group hopes to begin clinical trials in the next several years.
A Materials World
A short tricycle ride halfway around the ring from LS-CAT brings you to DND-CAT. Here the focus is the physical and material sciences. DND-CAT is one of the sectors at the APS that combines industrial and university partners.

Electromechanical technician Michael Bolbat rides a tricycle past LS-CAT, where APS research scientist David Smith works in one of the bays. Researchers use tricycles to get around the three-quarter-mile ring. Photo by Jim Prisching.
“DND-CAT gives students and faculty an elbow-to-elbow experience of working with industrial partners in a national lab setting,” says Michael Bedzyk. “Industrial research has a much longer-term view, with product output as its goal. We’re trying to do forefront basic science.
“At DND-CAT we have capabilities for looking at all types of materials, including polymers, semi-conductors, ceramics and energy materials like lithium-ion batteries,” Bedzyk goes on. “And we have instrumentation to allow our users to delve into any of those areas.”
Solar energy is one of the areas that Northwestern faculty and students explore at the Advanced Photon Source. Researchers are trying to find an efficient way to use the sun’s energy to convert water to storable hydrogen and oxygen — a kind of artificial photosynthesis, says Wasielewski, director of the Argonne-Northwestern Solar Energy Research Center, a collaboration between Northwestern, Argonne and three other universities.
“Hydrogen is a very good fuel,” he says. “It burns cleanly back into water, so it’s nonpolluting, carbon-neutral: All the good things you’d like to have.”
In natural photosynthesis, chlorophyll molecules in plants harvest light from the sun and then initiate the chemistry that stores that energy.
Instead of chlorophyll, Wasielewski and his colleagues use molecules that are ingredients in red automobile paint because, as Wasielewski points out, car paint has to be able to sit in the sun all day absorbing energy without breaking down.
The scientists pair these robust light-receptive molecules with a metallic catalyst that can react with water and break its chemical bonds to produce oxygen and hydrogen. The sun’s light then becomes the driver of that reaction, producing usable hydrogen fuel from water.
The trick, says Wasielewski, is getting the catalyst and the light receptor to work together. The researchers use both lasers and the APS beam to follow all of the chemical changes that happen to the catalysts.
The ANSER scientists are also pursuing novel designs for solar cells. The efficiency and usefulness of today’s solar cells are limited by their weight and cost. The ANSER group’s strategy is to make solar panels from very thin films of a light-absorbing organic polymer material that can be printed or even sprayed or painted on.
“These new materials would be very cheap, very robust and lightweight,” Wasielewski explains. “So you could incorporate them into packaging materials, into window shades, into all sorts of things you wouldn’t even dream of putting a solar panel on today.”
A solar cell is a sandwich: two electrodes with a filling of the active material between. “Electrical charges are generated in the meat of the sandwich,” continues Wasielewski, “and then they have to find their way into the bread — the electrodes.”
This is tricky, even when the sandwich is extremely thin. “If there’s a lot of disorder, the charges get trapped,” Wasielewski says. “We need to understand where they’re getting trapped.”
The group is trying to design systems of flat molecules that will stack themselves spontaneously between electrodes. A probe with the X-ray beam can tell the scientists just how well-ordered the layers of molecules are, whether parts of the stack have “fallen over.” Then they can rework their molecules. It is design at an incredibly minute level: “We do it literally atom by atom,” says Wasielewski.
Designing New Catalysts
Atomic-level construction is transforming the commercially gigantic field of catalyst design as well.
“Almost every material or chemical in modern life has a catalytic process somewhere in the making of it,” explains Weinberg College chemistry professor Peter Stair, who directs the Northwestern Institute for Catalysis in Energy Processes. “But our understanding of how many of them work is either poor or nonexistent.” Stair’s group designs new catalysts and then uses the beam to look inside a catalyzed chemical reaction while it happens.
One case of particular commercial importance is making ethane — a component of natural gas — into ethylene. Ethylene is used to make polyethylene plastic, which is, as Stair puts it, “the material for making almost everything,” from plastic bags to artificial hips.
The ideal catalyst has lots of surface area and very little “inside,” since it is only the atoms on the surface that participate in a reaction. This makes porous materials very good “platforms” for catalysts. The catalytic converter in a car, for example, is made from a honeycomb-like structure, with each cell providing surface area for the reaction.
The structures Stair and colleagues deal with are much smaller. “The material looks like sand,” says Stair. “It’s a powder.” Each grain is dappled with minute pores. As they build these tiny structures, X-ray diffraction measurements at the APS help them monitor the formation of the pores. Fine-tuning their size and shape helps control the reaction.
The porous “platform” becomes a catalyst when catalytic metal atoms are laid into the pores. But conventional methods of putting metal films onto such tiny structures end up clogging the pores rather than coating them.
So the scientists use a method called atomic layer deposition, which lets them lay down a single layer of atoms at a time. They can put down several layers in sequence, each with different atoms, designing and building up a catalytic film atom by atom.
“By controlling the sequence you can control the composition of the layer,” says Stair, “making a new composition for a catalyst.”
The scientists use the X-ray beam to watch the new catalyst in action. “We know that when the catalyst starts acting as a catalyst, it changes,” Stair says. “We need to understand how.” The beam can “see” the catalyst inside the reactor, he says. “It can tell us if the metals are changing, if the structure is changing. Nothing we have in our lab can do that. The APS can.”
Beyond Lithium Ion: Ramping Up Energy Storage
Kenneth Poeppelmeier and his colleagues at the Argonne-led Joint Center for Energy Storage Research use the APS to look inside a battery and observe the reactions as they occur, or in other words, as a battery is charged and discharged. JCESR is a Department of Energy–funded national partnership of universities, national laboratories and industry. Its stated mission: to develop new energy technologies that will “provide five times the energy storage at one-fifth the cost in five years” — a tall order.
One of the center’s major efforts is to develop batteries beyond lithium ion. Today’s electric cars — as well as many other devices in our lives — run on lithium-ion batteries. Poeppelmeier, the Charles E. and Emma H. Morrison Professor of Chemistry at Weinberg, and his colleagues aim to make batteries from new materials that they believe will increase the amount of energy a battery can store. Prime candidates for use as the cathode (the positive end of the battery) are magnesium and calcium intercalation compounds, which, while familiar elements in the periodic table, have never been demonstrated to work well in a battery before. The scientists plan to use the APS beam to do just that.
The battery itself will be small — a “coin cell” design similar to the button batteries used in consumer electronics today. The scientists want to know what is happening inside the battery as it’s working, not merely when it’s done its job and lies dissected on a lab bench.
“That’s where the APS comes in,“ says Poeppelmeier. The scientists will charge and discharge the battery while it sits in the X-ray beam and use X-ray diffraction to chart the changes to the structure of its components. This information will greatly speed up their investigations.
“This is an experiment that can be done best at the APS, where you have high-energy X-rays that can penetrate the battery’s casing, which allows the experiment to be carried out in situ,” he explains.

Lightning flashes across the night sky above the Advanced Photon Source at Argonne National Laboratory near Lemont, Ill. Courtesy of Argonne National Laboratory.
Beam Teams Collaborate Across Disciplines
A visitor to the research bays along the beamline will find it striking to see how collaborative and interdisciplinary work at the APS is.
A single research group might include participants from four or five universities, as well as Argonne’s staff scientists and technicians. Any one of the projects on the beamline could include input from chemists, physicists, biologists, computer scientists, applied mathematicians and experts in optics or lasers or X-ray microscopes or any of the myriad devices and technologies needed to take a project from question to answer.
“Many problems that are core to one discipline or another have already been tackled by people in that discipline,” observes Chris Jacobsen, an associate division director in the X-ray Science Division at the APS and a professor of physics in the Weinberg College. “Some of the most interesting problems that remain are the ones that lie at the intersection of fields.”
Jacobsen is the X-ray microscopy expert in a group studying the way diatoms, the tiniest marine organisms, trap carbon and affect both the ocean’s food chain and the planet’s climate. The project includes oceanographers, a specialist in making samples, computer scientists to help process the data, mathematicians to devise algorithms to improve the images reconstructed from the data, even a postdoc who is using video game technology to speed up the whole business of data manipulation.
“No one person can be fully expert in all these things,” Jacobsen says. “You have to bring in all these different skills to make progress.”
For students, exposure to this way of working can be eye opening. Jonathan Emery ’13 PhD, now a postdoc at Argonne, did research at the APS as a graduate student in Michael Bedzyk’s lab.
“I think collaboration allows you to do really good science,” he says. “When you know the guy right around the ring is an expert in just the problem you’re having, it’s really great to be able to go and have a conversation about it. You’re able to do that in this environment.” Adds doctoral student Leah Shoer, “Working at the APS gives you the idea that you’re not just a Northwestern scientist — you’re part of the larger scientific community.”
That community is international, and highly competitive, says Poeppelmeier. Experience with intense scientific teamwork is good preparation for students who will have to find a place in that world.
“The students are challenged,” Poeppelmeier says. “They have to up their game quite a lot.” But, he goes on, “We all have to up our game quite a lot in these collaborations. The faculty, the students — we have to learn new concepts. And sometimes you have to come up to speed on them pretty quickly and be able to communicate outside your area of expertise.”
When you couple the stimulation of collaboration with the best possible equipment at the APS, the result, Poeppelmeier says, is that the research being done by Northwestern faculty and students in collaboration with Argonne scientists is “at the highest level possible, and competitive with any place in the United States or the world. I think most scientists agree that this way of working is the future of science.”



 Facebook
Facebook Twitter
Twitter Email
Email